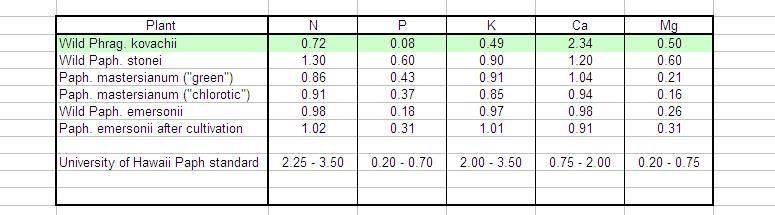
If you pile on the K they will stunt and become Ca deficient (no matter how much oystershell you put in the pot).
Just a couple of questions Rick.
What would you call ''piling''on the K. You often make this claim but how do you know there is an actual Ca deficiency? What are the symptoms? How would you prove the deficiency without leaf analysis if there are no obvious symptoms? Or are there? Please share them if there are....I don't think it is fare to make recommendations to others on a hunch without proof.
My kovachii has been recieving about 25 to 30 ppm K (at each watering) and similar N. Is that piling? I don't see any ''stunting'' though.
(I may reduce the total EC even further in the future down to about 15ppm N and K)
Ca deficiency often first manifests as browning root tips followed by root death. But I don't see that either. So what does the Ca deficiecy you speak of do? I don't think you can have good root growth AND a Ca deficiency AND vigorous young growth.
I believe you don't have to worry about the K factor. (with any PLANT at all for that matter let alone orchids, unless you are being rediculously foolish with your fertilizing habits) But if you keep the total EC low, keep the roots wet, and hold the pH around 7, kovachii will not have a Ca deficiency and grow as well as any other orchid. In fact it seems easier than many Paphs.
I also think the proplem with a lot of us is/was feeding the poor things when they are not receptive. I have discovered thet the worst thing you can do is to feed a plant when it is not growing. Stop feeding all together and eventually it gets going again. Only then can it take a balanced nutrition. Too much N stops root growth more surely than does too much K.